You are here: Home: NHLU 2 2005 : Mitchell R Smith, MD, PhD
| Mitchell R Smith, MD, PhD |
EDITED COMMENTS |
 Mantle cell lymphoma Mantle cell lymphoma
This disease was described for the first time in the early 1990s. We achieve high response rates to chemotherapy, but the disease always returns, and investigators have been searching for better treatments.
High-dose chemotherapy with stem cell transplant initially generated enthusiasm but that faded away because patients weren’t being cured; however, a low level of enthusiasm still exists and investigators continue to evaluate that approach.
MD Anderson published considerable data on the hyperfractionated cyclophosphamide, vincristine, Adriamycin and dexamethasone (CVAD) regimen followed by transplant. Single-institution trials always generate concern but over the years they demonstrated good results, and after rituximab was added to hyper-CVAD, the transplant really didn’t add much more. Currently, the best data is with the rituximab-hyper- CVAD (R-hyper-CVAD).
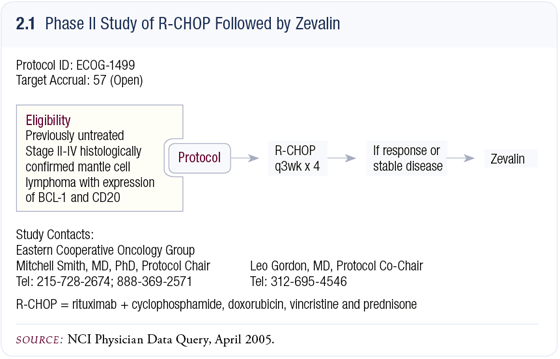
CHOP alone resulted in an average remission of approximately one year. Then Dana Farber ran a Phase II trial with R-CHOP and virtually every patient responded and the median duration of remission was approximately 16 months. So we can achieve high response rates, but can we maintain the remission? Transplant didn’t seem to do it, so we asked: If we can achieve high response rates with R-CHOP, will administering radioimmunotherapy take care of those remaining cells rather than waiting for relapse?
ECOG trial E1499: R-CHOP followed by rituximab + I-111- labeled ibritumomab tiuxetan (Zevalin®)
It took almost five years from the conception of this trial to actually opening it (2.1) due to significant concerns about the distribution of Zevalin while having rituximab on board. Thus, the FDA held up the trial for a while. The concern was that we might see excess toxicity, but we haven’t. Patients have recovered their counts so far, and we haven’t seen any unexpected toxicity. We can at least say that Zevalin can be administered within eight weeks of receiving rituximab with CHOP. We are measuring rituximab levels and don’t have that data yet, but we expect it to still be on board.
We still administer the “cold” rituximab with Zevalin, which raises another issue. If you look at the data, some cold rituximab is definitely needed when you administer Zevalin as a single agent. The radioactive antibody is actually a very small amount of protein, and if there’s a lot of circulating B-cells in the blood and spleen, they soak up the radioactive antibody.
If you scan a patient after giving radioactive antibody, with no cold antibody, it’s in the blood and the spleen and is rapidly cleared. It never actually gets to the lymph nodes where you want it. Administering the cold rituximab allows you to saturate those sites and allows the antibody to penetrate further into the lymph nodes.
However, if you already have circulating levels of rituximab, do you need this or not? We don’t really know. That’s the way the drug was approved, so we administered it in the approved fashion. How much cold rituximab is necessary? Whether patients received rituximab 50 mg/m2 or 250 mg/m2 didn’t seem to matter. We’re not seeing undue toxicity with the 250 mg/m2 added to the circulating levels, and we don’t believe it’s a problem to give extra rituximab.
Nonprotocol therapy for patients with mantle cell lymphoma
Based on the MD Anderson data, I usually discuss R-hyper-CVAD with younger patients. It’s an intensive regimen, and for many patients it’s questionable whether they will get through the treatment. Next I discuss R-CHOP, with the understanding that it will not cure them. Finally, we have a number of backup, second-line and biologically targeted therapies. Certainly, if patients don’t have a complete remission from R-CHOP, then autologous transplant — if the patient can tolerate it — may prolong the duration of remission, although it’s not likely to be curative.
Counseling patients about the risks and benefits of R-hyper-CVAD
Patients receiving R-hyper-CVAD are in the hospital for a fair amount of time receiving hyperfractionated cyclophosphamide followed by alternating methotrexate and cytarabine. They have a significant risk of infection during that time and they experience fatigue, so the therapy has a big impact on their ability to work and on their quality of life. In addition, the regimen carries a several percent risk of mortality and a significant risk of morbidity.
The incidence of neutropenia is high with this regimen, and neutropenic fever occurs in over 50 percent of the cases, even with growth factors. In patients who become neutropenic, I tend to use prophylactic antibiotics in an attempt to keep them out of the hospital, but a significant number of patients are still hospitalized with fever. I try to keep them on the treatment schedule at full dose, because if we reduce the dose, we compromise the result.
When treating a patient with R-hyper-CVAD for mantle cell lymphoma, I usually tell them it’s not considered curable today. A young, healthy patient who can get through this regimen has probably a 70 percent to 75 percent chance of being alive at two to three years, but their chance of being alive at five years is probably more like 20 percent to 25 percent. Of course, the hope is that we will develop new treatments during that period of time to deal with the expected relapse. We have some patients who are four or five years post-treatment, so a fraction of patients may be cured, but we can’t promise that at this point.
Role of radioimmunotherapy in patients with indolent lymphoma
When considering the treatment algorithms for low-grade lymphoma, obviously a wide range of options exists. Sometimes we agonize over the order in which to use them; however, over time we’ll probably use all of the treatments, and the order is probably not critical. Currently, I believe radioimmunotherapy should be considered after first relapse; whether it’s used as second-, third- or fourth-line therapy will depend on the patient. These are good treatments, and patients like them because it’s a targeted therapy and they complete treatment in a week.
Interesting data using Bexxar® (tositumomab + I-131 tositumomab) up front was recently published in the New England Journal of Medicine (Kaminski 2005). Obviously, this is not an approved use for the drug, and I don’t believe this should be done off study. In the accompanying editorial, Dr Connors warns that selection bias might have affected the results.
The median age of patients was the late forties, and some had slowly progressive disease so they may have had good risk features. This is not your typical elderly patient coming into the office with low-grade lymphoma. Some patients were older and it may apply to them as well, but we have to be careful about jumping to that conclusion.
The response rates to radioimmunotherapy have generally been in the 70 percent to 80 percent range in any line of therapy. In Kaminski’s data, the response rates were at least that good. The key was the duration of remission, which was quite long — a number of patients are out three, four, five years or longer without evidence of disease. Certainly, the potential for one week of therapy to induce a long remission and possibly cure a small group of these patients is exciting, but it’s too early to know.
Integrating rituximab with radioimmunotherapy
A number of ways to integrate radioimmunotherapy with rituximab are being studied with rituximab as a pretreatment or as a maintenance therapy. One of the concerns with the radioimmunotherapy data is that although the response rates are high, the response duration is disappointing. In most of the studies, the duration was 12 to 18 months.
One idea is to add rituximab maintenance to take advantage of the high response rate and try to prolong duration. Dr Hainsworth’s group has examined rituximab followed by rituximab/chemotherapy, followed by radioimmunotherapy in low-grade lymphoma (Shipley 2004).
The degree of bone marrow involvement can be a problem when considering radioimmunotherapy — if more than 25 percent is involved, the patient is ineligible. We could possibly expand the pool of eligible patients by cytoreduction and clearing the marrow. One way to do that would be with rituximab, if their disease is not refractory. One could consider using chemotherapy, but using rituximab to reduce the bone marrow tumor burden would allow patients to then receive radioimmunotherapy.
Select publications
| Dr Smith is the Director of the Lymphoma Service at Fox Chase Cancer Center in Philadelphia, Pennsylvania. |
|

