You are here: Home: NHLU 6 2005 : Craig Moskowitz, MD

| CD 1, Tracks 1-19 |
| Track 1 |
Introduction by Neil Love, MD |
| Track 2 |
Use of dose-dense chemotherapy
schedules in the treatment
of lymphomas |
| Track 3 |
Clinical trial of induction
R-CHOP-14 followed by ICE
consolidation chemotherapy |
| Track 4 |
Prognostic value of interim
restaging PET scans |
| Track 5 |
Ongoing clinical research
strategies evaluating
chemotherapy duration and
schedule |
| Track 6 |
Clinical use of PET imaging for
aggressive lymphomas |
| Track 7 |
Assuring quality control of
PET imaging |
| Track 8 |
Nodal sampling during
repeat biopsies |
| Track 9 |
Clinical research strategies in
diffuse large B-cell lymphoma |
| Track 10 |
Radioimmunotherapy in the
treatment of diffuse large
B-cell lymphoma |
|
| Track 11 |
Clinical treatment algorithm for
younger patients with mantle
cell lymphoma |
| Track 12 |
Clinical treatment algorithm for
older patients with mantle
cell lymphoma |
| Track 13 |
Development of novel agents for
mantle cell lymphoma in
clinical trials |
| Track 14 |
MD Anderson hyper-CVAD
regimen |
| Track 15 |
Clinical experience with the
proteasome inhibitor bortezomib |
| Track 16 |
Mechanism of action
of bortezomib |
| Track 17 |
FAV-ID-06 study: Idiotype-KLH conjugate versus placebo
following treatment with rituximab
in patients with follicular B-cell
non-Hodgkin’s lymphoma |
| Track 18 |
Selection of chemotherapy in
combination with rituximab for
indolent lymphoma |
| Track 19 |
Radioimmunotherapy as first-line
therapy for patients with
follicular lymphoma |
|
|
Select Excerpts from the Interview
 CD 1, Track 2 CD 1, Track 2
 DR LOVE: Would you provide an overview of research in dose-dense chemotherapy for lymphoma? DR LOVE: Would you provide an overview of research in dose-dense chemotherapy for lymphoma? |
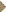 DR MOSKOWITZ: We have been administering dose-dense chemotherapy
to patients with untreated diffuse large B-cell lymphoma, peripheral T-cell
lymphoma or aplastic large cell lymphoma since 1993. Initially, we administered
induction therapy with 60 mg/m2 of doxorubicin and 1.4 mg/m2 of
vincristine, not capped (1.1). We repeated the imaging studies, and patients
who had an excellent response received consolidation therapy with high-dose
cyclophosphamide every two weeks times three — very similar to the dosedense
treatment with AC in breast cancer. DR MOSKOWITZ: We have been administering dose-dense chemotherapy
to patients with untreated diffuse large B-cell lymphoma, peripheral T-cell
lymphoma or aplastic large cell lymphoma since 1993. Initially, we administered
induction therapy with 60 mg/m2 of doxorubicin and 1.4 mg/m2 of
vincristine, not capped (1.1). We repeated the imaging studies, and patients
who had an excellent response received consolidation therapy with high-dose
cyclophosphamide every two weeks times three — very similar to the dosedense
treatment with AC in breast cancer.
The long-term follow-up was published last year in the Annals of Oncology (Portlock 2004). In general, we were not enamored with the high-dose cyclophosphamide
component, and at that time we were doing gallium scanning.
Patients who still had gallium-avid disease after the doxorubicin-based chemotherapy
rarely went into remission with cyclophosphamide.
Therefore, we developed a new strategy utilizing CHOP administered every
14 days. It was not an uncommon treatment program — it was also being
studied in a German lymphoma study group. In general, we administered
standard-dose CHOP every two weeks, and again we did not cap the vincristine
dose. We administered growth factors, usually on days six through 10,
and we found that the regimen was well tolerated.
The patients were a mixed population, but the long-term event-free survival
was more than 60 percent. The regimen took only 12 weeks to administer,
and at that time — in the pre-rituximab era — we considered using accelerated
chemotherapy for all patients with diffuse large B-cell lymphoma.
 DR LOVE: How was it tolerated in older patients? DR LOVE: How was it tolerated in older patients?
 DR MOSKOWITZ: We didn’t recommend it for older patients. However, in a
landmark paper published in Blood last year (Pfreundschuh 2004), the German
lymphoma study group reported that CHOP-14 administered for six cycles
is the standard treatment, at least in Germany, for older patients with diffuse
large B-cell lymphoma. It turned out to be the winner compared to CHOP
every 21 days or CHOP with etoposide. So it’s well tolerated in the older
patient population. DR MOSKOWITZ: We didn’t recommend it for older patients. However, in a
landmark paper published in Blood last year (Pfreundschuh 2004), the German
lymphoma study group reported that CHOP-14 administered for six cycles
is the standard treatment, at least in Germany, for older patients with diffuse
large B-cell lymphoma. It turned out to be the winner compared to CHOP
every 21 days or CHOP with etoposide. So it’s well tolerated in the older
patient population.
 CD 1, Track 3-4 CD 1, Track 3-4
 DR LOVE: When do you believe patients with lymphoma experience the maximum benefit from dose-dense chemotherapy? DR LOVE: When do you believe patients with lymphoma experience the maximum benefit from dose-dense chemotherapy? |
 DR MOSKOWITZ: We believe that if you can get the chemotherapy in on time
at full dose, patients will probably derive the maximal benefit early on in their
treatment. The definition of maximal benefit has been in a state of flux. DR MOSKOWITZ: We believe that if you can get the chemotherapy in on time
at full dose, patients will probably derive the maximal benefit early on in their
treatment. The definition of maximal benefit has been in a state of flux.
We are conducting a study at Memorial Sloan-Kettering right now that incorporates
rituximab-based treatment, dose-dense chemotherapy, and ifosfamide/carboplatin/etoposide (ICE) chemotherapy in the up-front setting. In order
to be eligible, a patient must have at least one risk factor in the age-adjusted
International Prognostic Index: advanced stage disease, elevated LDH or a
poor performance status.
With that background, we chose an induction regimen of R-CHOP administered
every 14 days. We previously piloted that regimen and published
those findings this year in Leukemia and Lymphoma (Halaas 2005). No difference
appeared between the side-effect profiles of R-CHOP every 14 days
and R-CHOP every 21 days. Once again, in a mixed population of patients,
long-term event-free survival approached 80 percent with diffuse large B-cell
lymphoma.
Thus far, our study has accrued approximately 60 patients. The primary
endpoint was to bring ICE — which is currently the most common secondline
regimen used in the United States — up front. We’ve administered it to
almost 700 patients with aggressive lymphoma and Hodgkin’s lymphoma, and
we believe it’s ready for prime time. So the standard treatment arm in this
particular study receives R-CHOP-14 for four cycles followed by three cycles
of ICE consolidation. In order to receive that treatment, a patient’s PET scan
must be negative after R-CHOP-14 has been administered four times.
 DR LOVE: What do we know about follow-up on patients from your study? DR LOVE: What do we know about follow-up on patients from your study?
 DR MOSKOWITZ: Of the first 60 patients, we had one patient who progressed
on R-CHOP-14, so we have 59 patients left. Of the 59 remaining patients,
40 had a negative PET scan after the R-CHOP-14. Of those 40 patients,
36 patients remain progression-free. Only four patients who had a positive
interim restaging PET scan progressed. DR MOSKOWITZ: Of the first 60 patients, we had one patient who progressed
on R-CHOP-14, so we have 59 patients left. Of the 59 remaining patients,
40 had a negative PET scan after the R-CHOP-14. Of those 40 patients,
36 patients remain progression-free. Only four patients who had a positive
interim restaging PET scan progressed.
It’s interesting that the positive predictive value of an interim restaging PET scan at this time is poor — it’s in the 30 percent range. That means it makes
no sense to change your treatment based on this interim restaging PET scan.
The other thing that’s interesting with this dose-dense treatment is that
diffuse large B-cell lymphoma turns out to be more than one disease. The
best way of thinking about it is that B-cell lymphoma derives from a certain
cell within the lymph node — we call that the cell of origin. To simplify,
B-cell lymphoma derives from either a germinal center B cell or a nongerminal
center B cell. Among patients who receive CHOP chemotherapy for
de novo diffuse large B-cell lymphoma, there’s evidence in the literature that
those whose cell of origin is of the germinal center do much better than those
whose cell of origin is of the nongerminal center (Hans 2004).
However, in this particular study, dose-dense, aggressive chemotherapy can
overcome the prognostic significance of the cell of origin, so patients with
nongerminal center-derived, diffuse large B-cell lymphoma have experienced
exactly the same benefits as patients whose cell of origin arose from the
germinal center.
Right now, the follow-up in the study is short — it’s only 18 months — but
the event-free survival is 87 percent. It’s a 100-patient study, and we’re on
patient 65. I am reluctant to present the data until we have accrued nearly
all of the patients. Considering the accrual trend, I suspect the data will be
presented during ASH 2006.
Select publications
|

