You are here: Home: NHLU 6 2005 : Anas Younes, MD

| CD 2, Tracks 11-27 |
| Track 11 |
Introduction by Dr Love |
| Track 12 |
MInT trial: CHOP-21, CHOEP-21,
MACOP-B and PMitCEBO with
and without rituximab in patients
with aggressive lymphomas |
| Track 13 |
Rituximab plus chemotherapy
as the standard of care for
patients with diffuse large B-cell
lymphoma |
| Track 14 |
Therapeutic strategies to improve
survival in diffuse large B-cell
lymphoma |
| Track 15 |
Increased acceptance of the
Follicular Lymphoma International
Prognostic Index (FLIPI) |
| Track 16 |
Potential curability of follicular
lymphoma |
| Track 17 |
Options for first-line therapy in
patients with follicular lymphoma |
| Track 18 |
Selection of patients for rituximab
monotherapy |
| Track 19 |
Selection of chemotherapy
regimens in combination with
rituximab |
|
| Track 20 |
Incorporation of radioimmuno-therapy
and transplant in the
treatment algorithm for follicular
lymphoma |
| Track 21 |
Role of rituximab as maintenance
therapy |
| Track 22 |
Novel clinical research strategies
in follicular lymphoma |
| Track 23 |
Clinical advances in the treatment
of mantle cell lymphoma |
| Track 24 |
Treatment of patients with mantle
cell lymphoma who relapsed after
R-hyper-CVAD |
| Track 25 |
Efficacy and toxicity comparisons
of R-hyper-CVAD and R-CHOP |
| Track 26 |
Community oncologists and
the treatment of non-Hodgkin’s
lymphoma |
| Track 27 |
Clinical trials evaluating novel
biologic agents for non-Hodgkin’s
lymphoma |
|
|
Select Excerpts from the Interview
 CD 2, Track 12 CD 2, Track 12
 DR LOVE: Would you provide an overview of the Mabthera International Trial (MInT) and some of the trials for patients who relapse? DR LOVE: Would you provide an overview of the Mabthera International Trial (MInT) and some of the trials for patients who relapse? |
 DR YOUNES: MInT was spearheaded by Michael Pfreundschuh from
Germany (Pfreundschuh 2004a, 2004b). It was truly an international trial
because it included investigators from Europe, Asia and South America. The trial specifically looked at the role of rituximab in combination with chemotherapy. The specific regimen was chosen by the investigator, and approximately
50 percent of the investigators used CHOP, while others — particularly
in Germany — used a CHOP equivalent such as CHOEP. DR YOUNES: MInT was spearheaded by Michael Pfreundschuh from
Germany (Pfreundschuh 2004a, 2004b). It was truly an international trial
because it included investigators from Europe, Asia and South America. The trial specifically looked at the role of rituximab in combination with chemotherapy. The specific regimen was chosen by the investigator, and approximately
50 percent of the investigators used CHOP, while others — particularly
in Germany — used a CHOP equivalent such as CHOEP.
MInT confirmed, in a randomized fashion, that the addition of rituximab to
CHOP or CHOP-like regimens improves outcomes specifically in terms of
complete remission rate and survival in patients who are younger than 60 years
of age (3.1).
Trials have also been conducted for patients with relapsed disease. One trial
from Memorial Sloan-Kettering reported on the use of rituximab with isofosfamide/carboplatin/etoposide (ICE) chemotherapy (Kewalramani 2004). This
was not a randomized trial; it was a Phase II trial, but it compared data with
previous experience using ICE chemotherapy — sequential trials from the
same institution using similar eligibility criteria. They showed that the addition
of rituximab to salvage therapy like ICE improves the complete remission rate.
Improving complete remission rate prior to transplant is a very important
prognostic factor, because patients who receive transplant during complete
remission have a better outcome and survival than patients who are not in
complete remission at the time of transplantation.
We had similar outcomes in our institution using a regimen of paclitaxel,
topotecan and rituximab (TTR). We utilized the regimen for patients with
diffuse large B-cell lymphoma who failed to respond to front-line regimens
— they were treated with TTR at first or second relapse. We’ve seen a similar
improvement in overall response rate and an improvement in complete remission
rate, making these patients better candidates for stem cell transplantation.
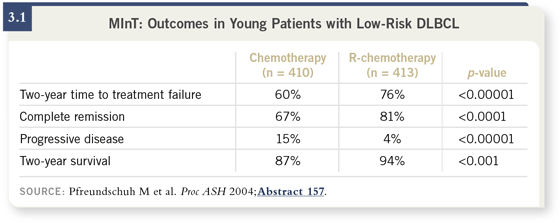
 CD 2, Track 13 CD 2, Track 13
 DR LOVE: What are your thoughts about dose-dense chemotherapy in patients with lymphoma? DR LOVE: What are your thoughts about dose-dense chemotherapy in patients with lymphoma? |
 DR YOUNES: Dose-dense therapy is an important concept that is now gaining
some appeal, especially for solid tumors. In non-Hodgkin’s lymphoma, Michael Pfreundschuh spearheaded a randomized trial — published in Blood
— that compared CHOP-14 and CHOP-21 with or without etoposide
(Pfreundschuh 2004c, 2004d). The interesting thing about the data was — at
least in the elderly patients — the dose-dense 14-day regimen was superior
to the 21-day regimen (3.2). However, these trials were conducted before the
incorporation of rituximab, so we don’t know if R-CHOP-14 is superior to
R-CHOP-21. Currently, randomized trials are investigating this question, but
we don’t know the results yet. DR YOUNES: Dose-dense therapy is an important concept that is now gaining
some appeal, especially for solid tumors. In non-Hodgkin’s lymphoma, Michael Pfreundschuh spearheaded a randomized trial — published in Blood
— that compared CHOP-14 and CHOP-21 with or without etoposide
(Pfreundschuh 2004c, 2004d). The interesting thing about the data was — at
least in the elderly patients — the dose-dense 14-day regimen was superior
to the 21-day regimen (3.2). However, these trials were conducted before the
incorporation of rituximab, so we don’t know if R-CHOP-14 is superior to
R-CHOP-21. Currently, randomized trials are investigating this question, but
we don’t know the results yet.
 DR LOVE: At this time, do you think it’s rational to utilize R-CHOP-14 in
the clinical setting, particularly in patients with poor prognostic factors? DR LOVE: At this time, do you think it’s rational to utilize R-CHOP-14 in
the clinical setting, particularly in patients with poor prognostic factors?
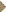 DR YOUNES: I don’t think it’s wrong to use R-CHOP-14 in selected patient
populations, simply because it’s not more toxic, at least based on the Pfreundschuh
data. We would not be harming patients, but whether or not it’s more
beneficial is yet to be determined in a randomized study. DR YOUNES: I don’t think it’s wrong to use R-CHOP-14 in selected patient
populations, simply because it’s not more toxic, at least based on the Pfreundschuh
data. We would not be harming patients, but whether or not it’s more
beneficial is yet to be determined in a randomized study.
 CD 2, Track 17 CD 2, Track 17
 DR LOVE: Would you discuss your approach to patients with follicular lymphoma who present for first-line therapy? DR LOVE: Would you discuss your approach to patients with follicular lymphoma who present for first-line therapy? |
 DR YOUNES: It’s complicated, particularly when you discuss these issues with
the increasing number of educated patients. They’re aware of what’s available
— a large menu of options that are all reasonable. But the majority of trials
never made head-to-head comparisons to determine whether one treatment is
better than the other, and that’s creating confusion for the patients. We know right now that when you add rituximab to a front-line regimen for patients
with advanced-stage follicular lymphoma, you improve outcomes in terms
of duration of remission and the percentage of patients whose disease enters
remission. Most oncologists believe this will eventually translate into improved
survival. DR YOUNES: It’s complicated, particularly when you discuss these issues with
the increasing number of educated patients. They’re aware of what’s available
— a large menu of options that are all reasonable. But the majority of trials
never made head-to-head comparisons to determine whether one treatment is
better than the other, and that’s creating confusion for the patients. We know right now that when you add rituximab to a front-line regimen for patients
with advanced-stage follicular lymphoma, you improve outcomes in terms
of duration of remission and the percentage of patients whose disease enters
remission. Most oncologists believe this will eventually translate into improved
survival.
What’s the optimal combination — chemotherapy with rituximab? No one
knows. A large menu of combination chemotherapy regimens is available that
you can combine with rituximab, or you can use rituximab alone. Furthermore,
information is emerging about the use of radioimmunotherapy up front
— at least in the experimental setting — that can demonstrate effectiveness
perhaps as good as combination chemotherapy but with shortened duration of
treatment and potentially fewer potential side effects (Kaminski 2005).
I am pro-clinical trial participation. Although I discuss the different options
that may be used off protocol, I tend to encourage patients to participate in
the clinical trials. Outside clinical trials, I use one of three combinations,
depending on the patient’s situation and preference. It’s a mutually agreeable
treatment plan. I tend to use R-CHOP, R-FND or R-CVP. I extensively
discuss the pros and cons of these regimens with the patients and their
families, and then we reach a mutual agreement. I think the majority of oncologists
in North America use R-CHOP or R-CVP for patients with advancedstage
follicular lymphoma.
Select publications
|

